What is ANVISA?
ANVISA was established in 1999 under Law No. 9.782 with the primary objective of promoting and protecting public health by regulating products and services subject to health surveillance. In Brazil, the registration of drugs and manufacturing sites with the National Health Surveillance Agency, ANVISA (Agência Nacional de Vigilância Sanitária), is a crucial step in ensuring the safety, efficacy, and quality of pharmaceutical products. Understanding the intricacies of this regulatory process is essential for companies seeking to bring drugs to market and operate manufacturing plants in Brazil.
Key Functions of ANVISA

Impact of ANVISA
Certified Manufacturers: Upholding Quality Standards
ANVISA oversees a network of certified manufacturers responsible for producing a diverse array of health-related products, including pharmaceuticals, medical devices, cosmetics, and food supplements. These manufacturers undergo stringent evaluations to obtain ANVISA certification, demonstrating compliance with Good Manufacturing Practices (GMP) and other regulatory requirements.
Approved Plants and Sites: Pillars of Reliability
Manufacturers operate within approved plants and sites, which are subject to regular inspections by ANVISA to verify compliance with safety and quality standards. ANVISA’s approval signifies that these facilities meet the necessary criteria for ensuring the integrity and reliability of the production process, from raw material sourcing to finished product distribution.
Reliable Suppliers and Exporters: Ensuring Traceability and Accountability
ANVISA oversees suppliers and exporters, ensuring that they uphold the same rigorous standards as manufacturers. By monitoring the entire supply chain, ANVISA ensures traceability and accountability, from the sourcing of raw materials to the distribution of finished products, safeguarding against potential risks to public health.
Proactive Enforcement: Mitigating Risks and Ensuring Compliance
ANVISA’s proactive enforcement measures include regular inspections, audits, and surveillance activities to identify and address potential risks promptly. Companies found to be non-compliant face stringent penalties, including fines, sanctions, and product recalls, reinforcing the importance of adherence to regulatory requirements.
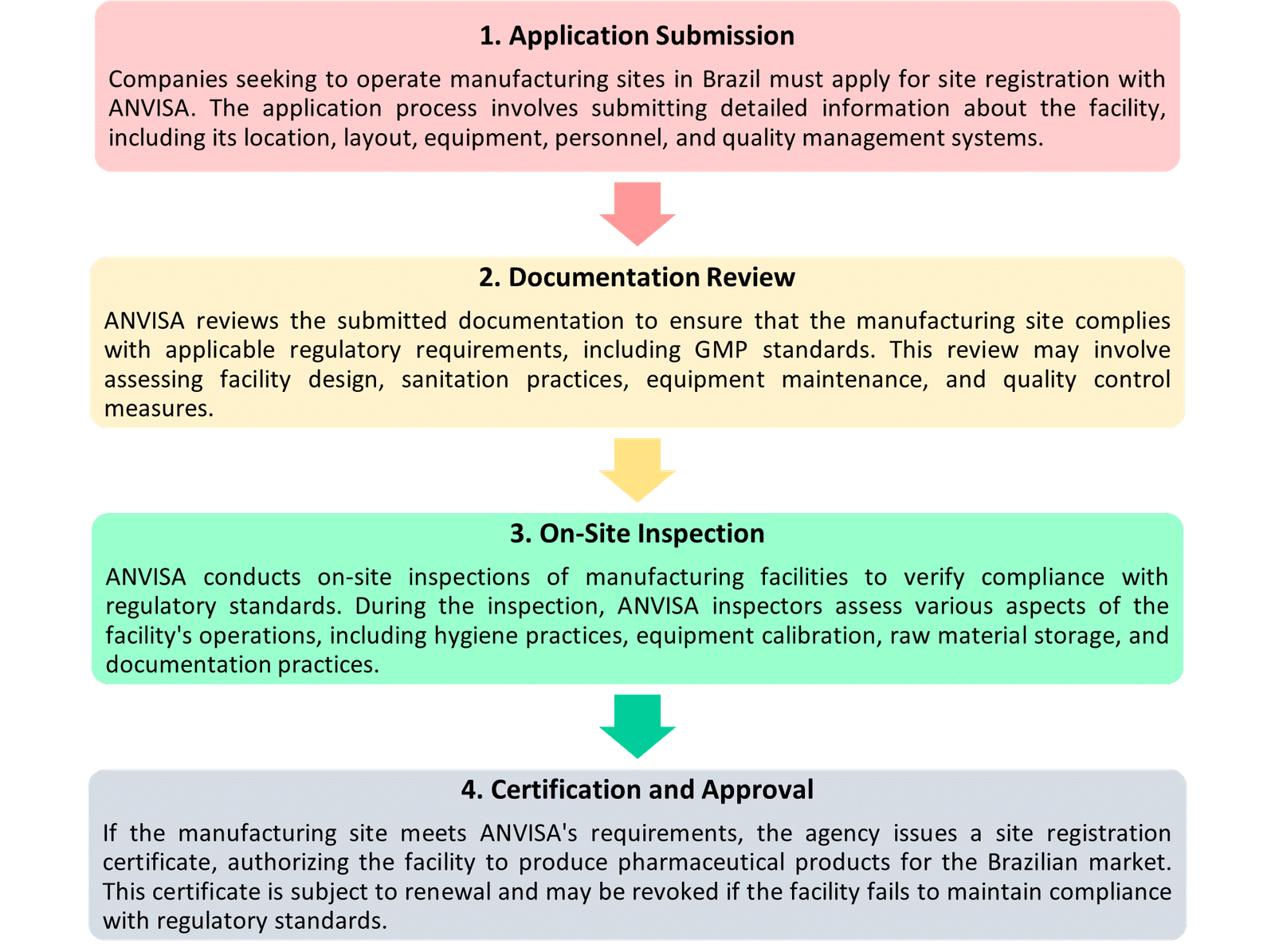
Site Registration Process for Approval of Manufacturing Plants
Drug Registration Process for ANVISA Approved Manufacturer

Brazil is the largest pharmaceutical market in South America with a CAGR of 10.6%. With the rise in globalization, pharmaceutical companies are keen on launching their products in the Brazilian market. Though the market has established a regulatory system, it’s highly complex. In order to register a product in Brazil, one needs approvals from ANVISA which accepts the dossier in eCTD format and is primarily categorized as New Drugs, Branded, and Non-Branded Generics.
New Drugs are innovative products whose safety and efficacy are been proven by various non-clinical and clinical trials.
Branded Generics: these products are similar to already registered products, having same concentration, dosage form, therapeutic value and can only differ in size, labeling, and packaging and should be determined by their trade names.
Non-Branded Generics (Similar Drugs), these are in all way similar to all registered drugs with ANVISA and primarily can be used interchangeably.
Different Steps of Drugs Registration in Brazil

A. Structure of Registration Dossier
In Brazil the registration dossier is structured in eCTD format and it has two main parts:
Part 1- Administrative Part:
It corresponds to Module 1 and includes compilation of all administrative data and local information
Part 2 – Technical Part:
It corresponds to Module 2, Module 3, Module 4 and Module 5 that include summaries of pre-clinical studies; CMC information; nonclinical data and reports; and clinical information and reports.
Content of Registration Dossier
Dossier Content |
New Drug |
Branded Generic Drug |
Similar Drug |
|---|---|---|---|
| Module 1 | |||
| Sanitary license of local representative | ✔ | ✔ | ✔ |
| Local representative operating authorization | ✔ | ✔ | ✔ |
| Registration of local Pharmacist at professional counsil | ✔ | ✔ | ✔ |
| Petition form | ✔ | ✔ | ✔ |
| Justification for product registration | ✔ | ✔ | ✔ |
| Labeling of different presentations | ✔ | ✔ | ✔ |
| Previous Communications with ANVISA | ✔ | ✔ | ✔ |
| CPP issued from the HA in country of origin | ✔ | ✔ | ✔ |
| GMP certificates issued from the HA in country of origin | ✔ | ✔ | ✔ |
| GMP certificates granted by ANVISA | ✔ | ✔ | ✔ |
| Registration status worldwide | ✔ | ✔ | ✔ |
| Pharmacovigilance data | ✔ | ✔ | ✔ |
| Product labeling in portuguese | ✔ | ✔ | ✔ |
| TSE information | ✔ | ✔ | ✔ |
|
Module 2 |
|||
| 2.1 Table of contents of Module 2 | ✔ | ||
| 2.2 Introduction | ✔ | ||
| 2.3 Quality Overall Summary | ✔ | ||
| 2.4 Non-clinical Overview | ✔ | ||
|
2.5 Clinical Overview |
✔ | ||
| 2.6 Non-clinical | ✔ | ||
| 2.7 Clinical Summary | ✔ | ||
|
Module 3 |
|||
| 3.1 Table of contents of Module 3 | ✔ | ✔ | ✔ |
| 3.2 Body of data | ✔ | ✔ | ✔ |
| 3.2.1 Drug Substance | ✔ | ✔ | ✔ |
| 3.2.2 Drug Product | ✔ | ✔ | ✔ |
| 3.3 Literature references used in Module 3 | ✔ | ✔ | ✔ |
|
Module 4 |
|||
| 4.1 Table of contents of Module 4 | ✔ | ||
| 4.2 Study reports | ✔ | ||
| 4.2.1 Pharmacology | ✔ | ||
| 4.2.2 Pharmacokinetics | ✔ | ||
| 4.2.3 Toxicology | ✔ | ||
| 4.3 Literature references used in Module 4 | ✔ | ||
|
Module 5 |
|||
| 5.1 Table of contents of Module 5 | ✔ | ||
| 5.2 Tabular listing of all clinical studies | ✔ | ||
| 5.3 Clinical study reports | ✔ | ||
| 5.3.1 Reports of biopharmaceutic studies | ✔ | ||
| 5.3.2 Reports of human pharmacokinetic (PK) studies | ✔ | ||
| 5.3.3 Reports of human pharmacodynamic (PD) studies | ✔ | ||
| 5.3.4 Reports of efficacy and safety studies | ✔ | ||
| 5.3.5 Reports of post-marketing experience | ✔ | ||
| 5.3.6 Case report forms and individual patient listings | ✔ | ||
| 5.4 Literature references used in Modulo 5 | ✔ | ||
| BE Studies | ✔ | ✔ | |
ANVISA Review Process of Registration Dossier
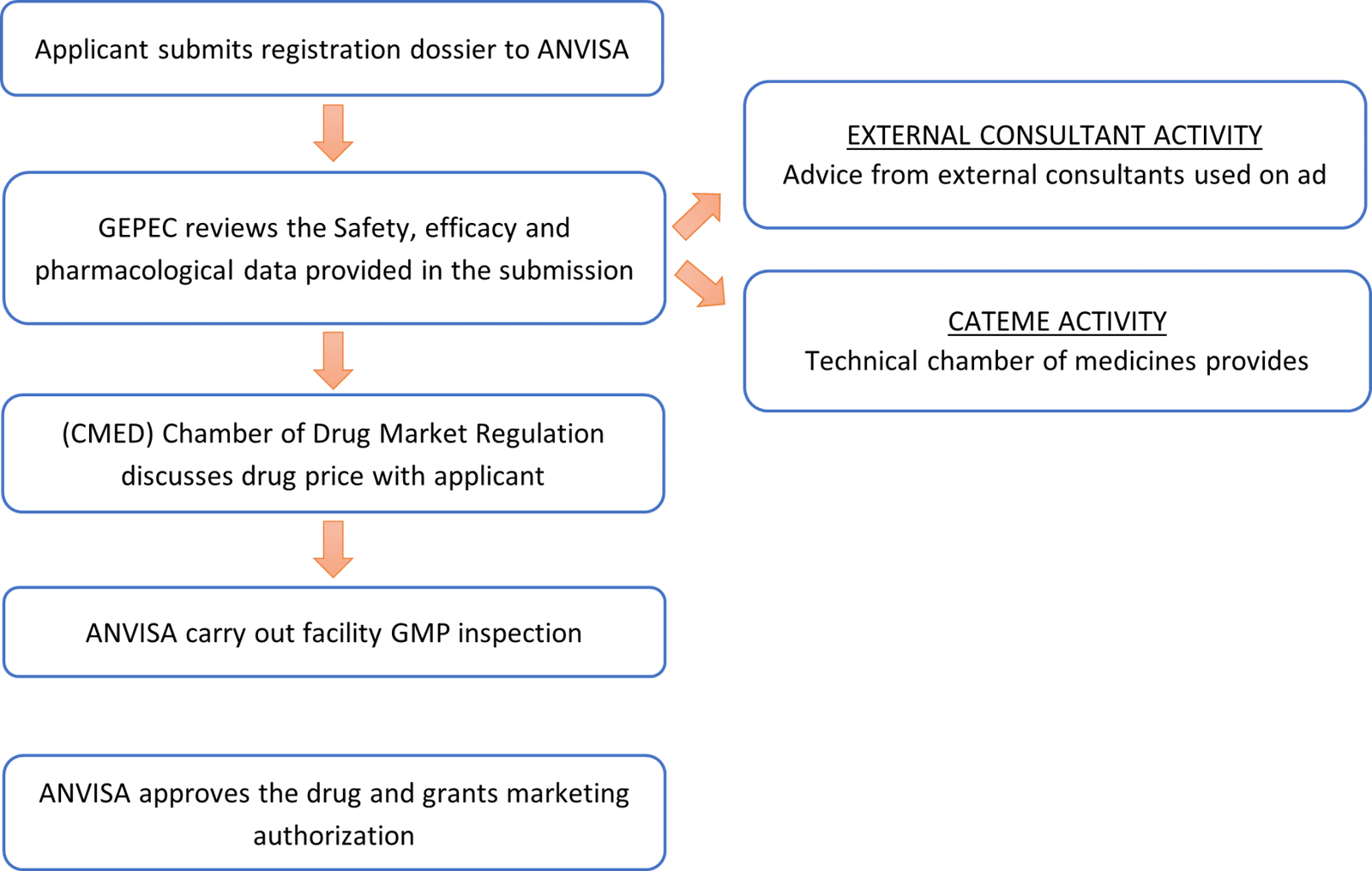
B. ANVISA GMP Inspection of Manufacturing Sites
- Content of the application for GMP inspection:
- Petition form completed, stamped and signed
- Valid GMP certificate issued by the health authority of the country of origin. Certificates issued in English or Spanish will be accepted without the need of a sworn translation.
- Plant Master File – AMP or Site Master File – SMF
- Periodical Product Review (RPP)
- Inspection Report from other health authorities in country of origin, if available
- Timelines for ANVISA inspection:
- ANVISA inspection of manufacturing site occurs approximately 6 months after submitting the request for inspection to ANVISA
- ANVISA issues the Good Manufacturing Practice certificate to company 45 to 60 days after inspection
C. Requirements for Local Testing
- The importer located in Brazil is responsible for performing complete quality control tests of finished products in accordance with the specifications and test methods registered in Brazil with ANVISA.
- The testing frequency depends on the number of shipments made to Brazil each year:
- Importation of > 8 shipments/year of each drug samples of 2 batches/ year to be tested
- Importation of ≤ 8 shipments /year of each drug samples of 2 batches/ every 2 years to be tested
- The time required for each local testing depends on the product’s specifications that need to be tested.
Registration Fees & Timelines to be ANVISA Approved Manufacturer
The timelines for drugs’ registration and post-approval changes are here below:
| PRIORITY REVIEW | STANDARD REVIEW | ||
| Registration | Post-approval Changes | Registration | Post-approval Changes |
| 120 Days | 60 Days | 365 Days | 180 Days |
| Classification |
Timeline for approval (months) |
Fees (USD) |
| New Drug | 12 – 14 | 2700 – 27000 |
| Branded Generic Drug | 6 -8 | 2000 |
| Similar Drug | 8 – 12 | 7000 |
Conclusion
Navigating ANVISA’s drug and site registration process is a complex yet essential aspect of bringing pharmaceutical products to market in Brazil. By adhering to regulatory requirements, companies can ensure the safety, efficacy, and quality of their products, ultimately contributing to the health and well-being of Brazilian consumers. With thorough preparation, diligent documentation, and compliance with GMP standards, companies can successfully navigate the regulatory landscape and obtain ANVISA approval for drug registration and manufacturing site operation.
References: Brazilian Health Regulatory Agency (Anvisa)
This article was crafted under the expert guidance of Ms. Ritika Patel, showcasing her invaluable contribution to its preparation and quality. – Team Conical Pharmaceuticals













