What is NAFDAC?
The National Agency for Food and Drug Administration and Control (NAFDAC) was established by Decree No. 15 of 1993 as amended by Decree No. 19 of 1999 and now the National Agency for Food and Drug Administration and Control Act Cap N1 Laws of the Federation of Nigeria (LFN) 2004 to regulate and control the manufacture, importation, exportation, distribution, advertisement, sale and use of Food, Drugs, Cosmetics, Medical Devices, Packaged Water, Chemicals and Detergents (collectively known as regulated products). The agency was officially established in October 1992.
Functions of NFADC

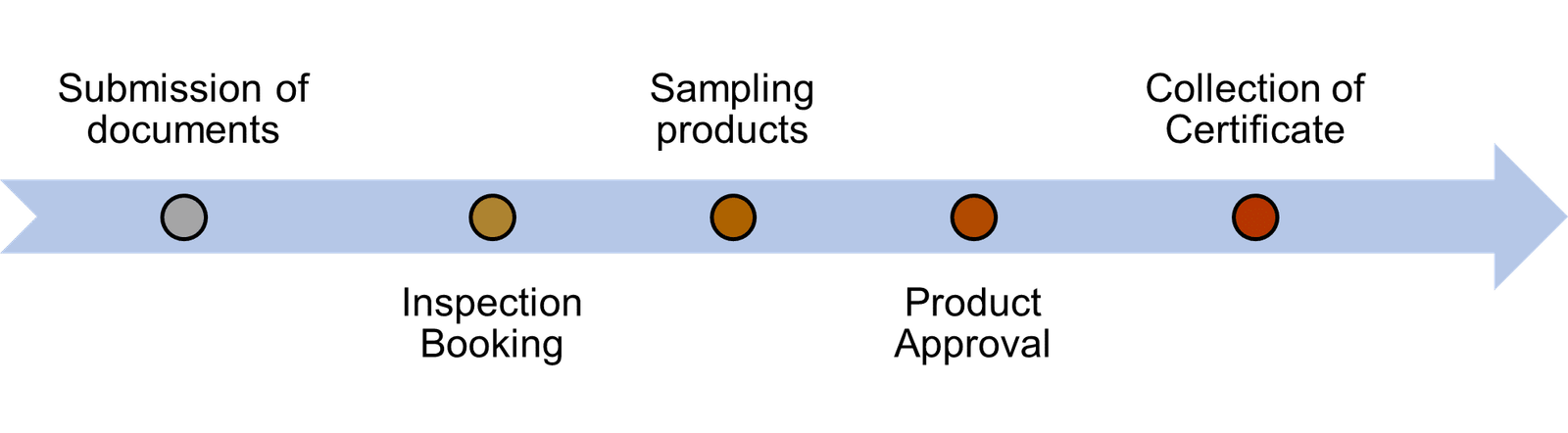
To be NAFDAC Approved Manufacturer in India, this Steps must be followed
Step 1: Submission of documents
To complete the registration process, submit a fully filled form along with proof of registration with the Corporate Affairs Commission (CAC), business name, and incorporation. Include a site use agreement (if applicable), trademark approval evidence, and three product labels. Also, provide a fumigation certificate, payment receipt for production inspection and laboratory analysis, and a food handlers’ or medical fitness certificate for production staff, covering sputum, stool, urinary and Hepatitis B tests.
Step 2: Inspection Booking
In booking for your facility inspection, you are given the privilege to pick a most convenient date for your inspection.
Step 3: Sampling products
Samples of products are collected during facility inspection and sent to the laboratory for analysis.
Note: Samples are taken when GMP/GHP is satisfactory.
Step 4: Product Approval
The products are schedule for approval upon satisfactory Good Manufacturing Practice (GMP) and laboratory report.
Step 5: Collection of Certificate
Company is notified to collect their certificate
Product registration process for NAFDAC Approved manufacturer in India
Content of Registration Dossier
Common Technical Document (CTD) Format
| Sr. No | Title and Main Section Headings |
|
Module 1: Administrative and Product Information |
|
| 1.0 | Cover Letter |
| 1.1 | Table of Contents (Modules 1 to 5) |
| 1.2 | Application Information |
| 1.3 | Product Information Regional |
| 1.4 | Summaries Electronic Review |
| 1.5 | Documents |
| 1.6 | Product Sample(s) (if available at the time of submission) |
| 1.A | Appendix |
|
Module 2: Common Technical Document (CTD) Summaries |
|
| 2.1 | CTD Table of Contents (Modules 2 to 5) |
| 2.2 | CTD Introduction |
| 2.3 | Quality Overall Summary |
| 2.4 | Nonclinical Overview |
| 2.5 | Clinical Overview |
| 2.6 | Nonclinical Written and Tabulated Summaries |
| 2.7 | Clinical Summary |
|
Module 3: Quality |
|
| 3.1 | Table of Contents of Module 3 |
| 3.2 | Body of Data |
| 3.3 | Literature References |
|
Module 4: Nonclinical Study Reports |
|
| 4.1 | Table of Contents of Module 4 |
| 4.2 | Study Reports |
| 4.3 | Literature References |
|
Module 5: Clinical Study Reports |
|
| 5.1 | Table of Contents of Module 5 |
| 5.2 | Tabular Listing of All Clinical Studies |
| 5.3 | Clinical Study Reports |
| 5.4 | Literature References |
Note: Module 4: Not required for generic products
Module 5: Bioequivalence or Biowaiver required for generics as applicable
Validity of NAFDAC Approved product certificate
5 (Five) years. This may be renewed for subsequent periods of 5 (Five) years each.
Timelines for Plant Approval and NAFDAC Product Approval
|
Application |
Timeline for approval |
| Submission of Application | 0 days |
| Verification of documents | 10 days |
| Facility Inspection/Sampling | 10 days for Food |
| 20 days for Drugs | |
| Laboratory Analysis | 30 days for Food |
| 20 days for Drugs | |
| Final Vetting | 10 days |
| Approval Meeting/Issuance of NAFDAC registration number | 20 days |
Charges and Fees for NAFDAC Product Approval
|
Product Type |
Registration Fee |
| Pharmaceuticals | ₦70,000 |
| Orphan Drugs | ₦25,000 |
| International inspection fees | ₦10,500 |
Challenges for getting NAFDAC Approval for Plant and product

Conclusion
In conclusion, obtaining NAFDAC approval is crucial for any NAFDAC approved plant or manufacturer looking to succeed in the Nigerian market. Becoming a NAFDAC certified manufacturer ensures that your products meet the highest safety and quality standards, which not only boosts consumer trust but also strengthens your position as a reliable supplier and exporter. By navigating the necessary steps and fulfilling regulatory requirements, NAFDAC approved manufacturers can confidently expand their reach, knowing that their products are certified, approved, and ready for both local and international markets.
This article was crafted under the expert guidance of Ms. Nidhi Patel, showcasing her invaluable contribution to its preparation and quality. – Team Conical Pharmaceuticals