Describe PPB Kenya
The Pharmacy and Poisons Board of Kenya is referred to as PPB Kenya. It is the regulatory organization in Kenya that oversees the registration, management, and control of medical devices, pharmaceuticals, and healthcare providers. Before these goods may be sold and utilized in the nation, the PPB makes sure they fulfil acceptable requirements for efficacy, safety, and quality. In order to maintain public health standards, the PPB also supervises the licensing and regulation of pharmacies, pharmaceutical producers, and other healthcare facilities.
Mission
To protect and promote the health of the public by regulating the profession of pharmacy and ensuring access to quality, safe, efficacious and affordable medical products and health technologies.
Vision
To be a global leader in promoting and protecting public health.
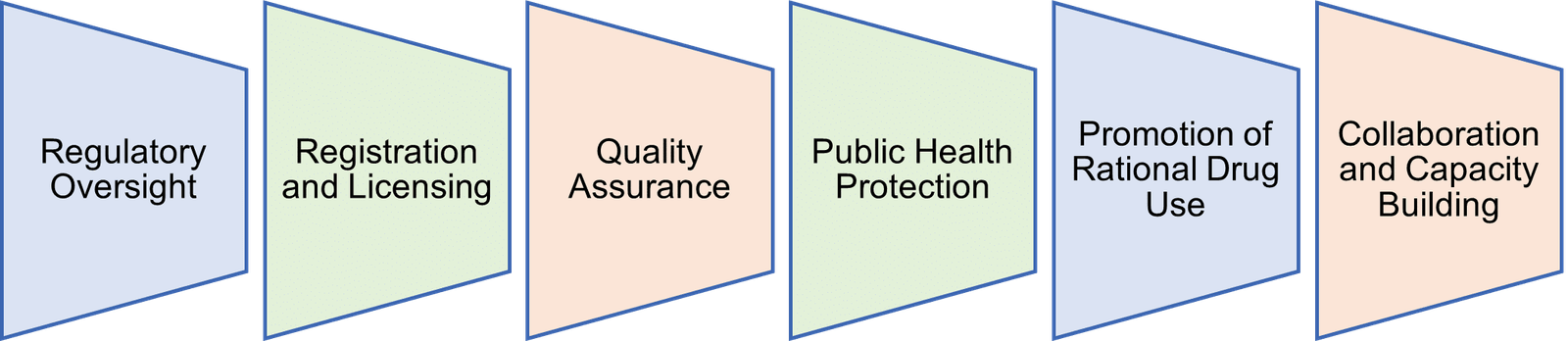
Our Mandate
The Board oversees the manufacture and trade of pharmaceuticals and poisons, as well as the practice of pharmacy.
In order to protect consumers as intended by Kenya’s drug laws, the Board seeks to implement the necessary regulatory measures to ensure the highest standards of safety, efficacy, and quality for all drugs, chemical substances, and medical devices, whether they are produced locally, import, export, distributed, sold, or used.
Objective
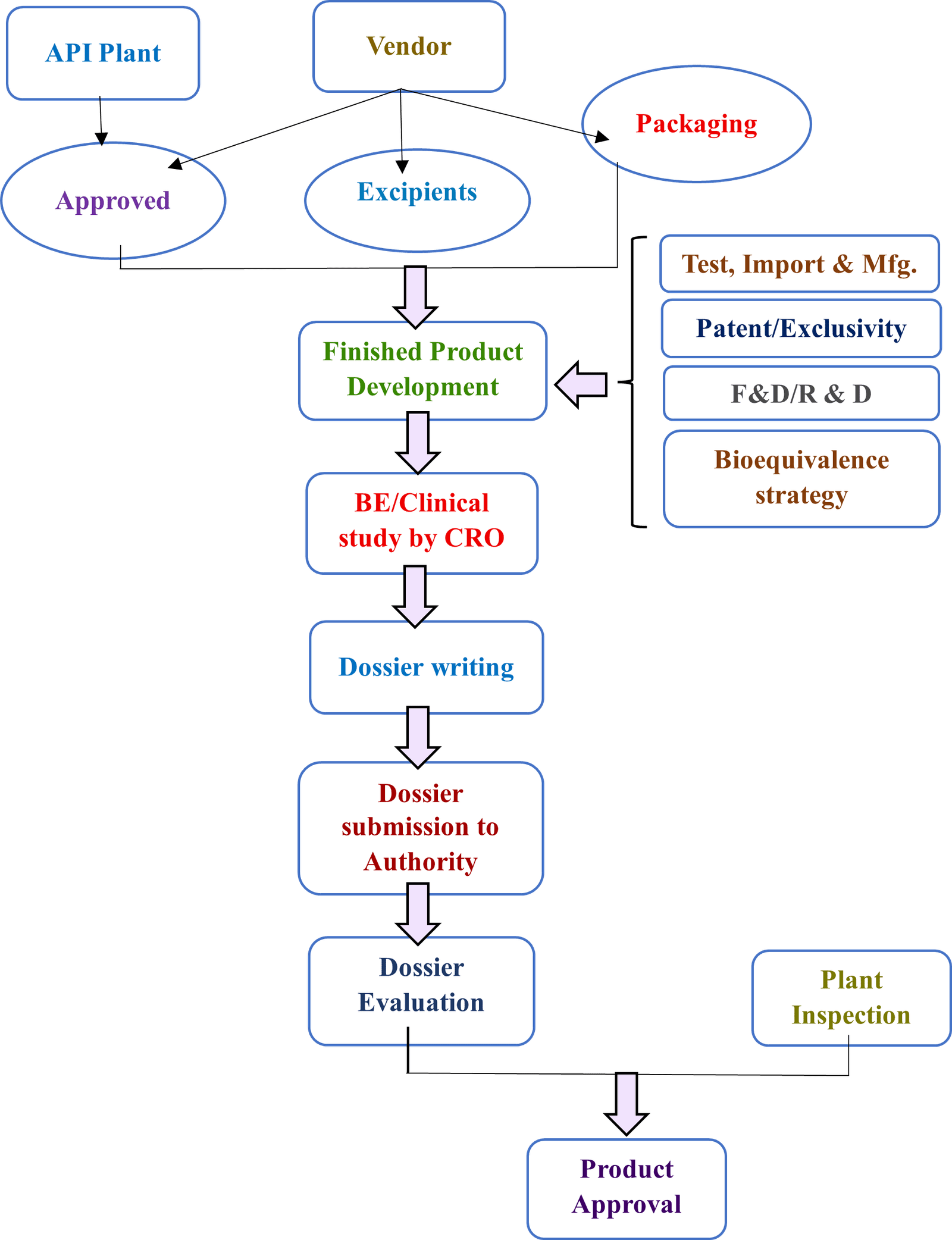
To be PPB Approved Manufacturer in India, this process must be followed
| Process | Timelines | Responsibility |
 |
1 Day | Applicant/PPB |
| Within 7 days of receiving application | The Board | |
| 0 Days | The Applicant | |
| Within 15 days of receiving application | The Board | |
| 14 Days from first reviewer approval | The Board | |
| Within 7 days of Inspection | The Board | |
| Within 15 days of receiving of inspection | The Board | |
| Downloadable immediately after second reviewer approval | The Board |
Being a PPB approved Site registration and licensing requirements
Regulatory Authority : Pharmacy and Poisons Board (PPB)
Website of regulatory Authority : http://pharmacyboardkenya.org
Fees for drug Registration : USD 1000
Normal time taken for registration : 12 Months
Registration Requirement [Dossier Format] : CTD
Whether plant inspection is mandatory : Yes
Requirement of local agent/ Subsidiary : Local agent is sufficient
New product registration process to be PPB approved manufacturer in India
Application for New Registration
A new application for registration shall include submission of:
- Two dully filled application forms (Original and duplicate) and an electronic copy (a summary of the dossier contents) in MS Word on a CD-ROM of modules 1 and 2 only including their supporting documents.
- Three (3) samples of the smallest commercial pack(s) from one batch with batch certificates of analysis.
- An original Certificate of Pharmaceutical Product (WHO Format) on official papers of the issuing competent drug regulatory authority.
- A site master file in case the product is manufactured at a plant(s) not inspected and approved by PPB.
- Non-refundable application fee for registration of medicines in Kenya and GMP inspection fees for facilities not yet inspected by PPB.
Applications for PPB Approved Product Renewal Registration
Applications for renewal of registration shall be made at least 3 months before the expiry of existing registration by submitting the following:
- Dully filled in application form for renewal of registration.
- Batch Manufacturing Record (BMR) of a real batch manufactured within at most six months before the submission of the application.
- Submit Periodic Safety Update Reports (PSUR).
- Proof of interchange ability for generics as explained in Module 5.
- Any other requirements that the Board may determine.
- Three (3) samples of the smallest commercial pack(s) from the same batch along with batch certificates of analysis.
- A site master file in case the product is manufactured at a plant(s) not inspected and approved by PPB.
- Non-refundable application fee for registration of medicines in Kenya and GMP inspection fees for facilities not inspected and approved by PPB, GMP department.
Application for Variation PPB Approved product
All applications for variation to a registered product shall be made according to requirements stipulated in the PPB Application Guideline for Variation of Registered Medicinal Products also available the PPB offices.
Evaluation of new applications
After the application is received, it will be handled in full within a year. Any further data that is needed must be provided by the applicant within six months. Should further time be needed, a formal request needs to be made.
Validity of PPB Approved product
A registration certificate will be valid for 5 years unless significant changes are made to the approved application data.
General Requirements for dossier Preparation
Common Technical Document is divided into five modules:
- Module 1: Administrative Information & Prescribing Information
- Module 2: Common Technical Document Summaries
- Module 3: Quality
- Module 4: Nonclinical Study Reports
- Module 5: Clinical Study Reports
The Annexes are:
- Annex I: Application for Registration of a Drug
- Annex II: Model Stability Report for Active Pharmaceutical Ingredients (APIs)
- Annex III: Model Stability Report for Finished Pharmaceutical Products
- Annex IV: Summary Product Characteristics
Costs associated with PPB approved plant in India for product registration
| Sr. No | Applications various forms | Fees |
| 1. | Plant Inspection fees | US$ 4000 |
| 2. | Application for registration of a pharmaceutical product | US$ 1000 |
| 3. | Applications for Approved Product Renewal Registration | US$ 500 |
| 4. | Variations | US$ 200 |
| 5. | Replacement of a Certificate | US$ 100 |
| 6. | Appeal fee | US$ 300 |
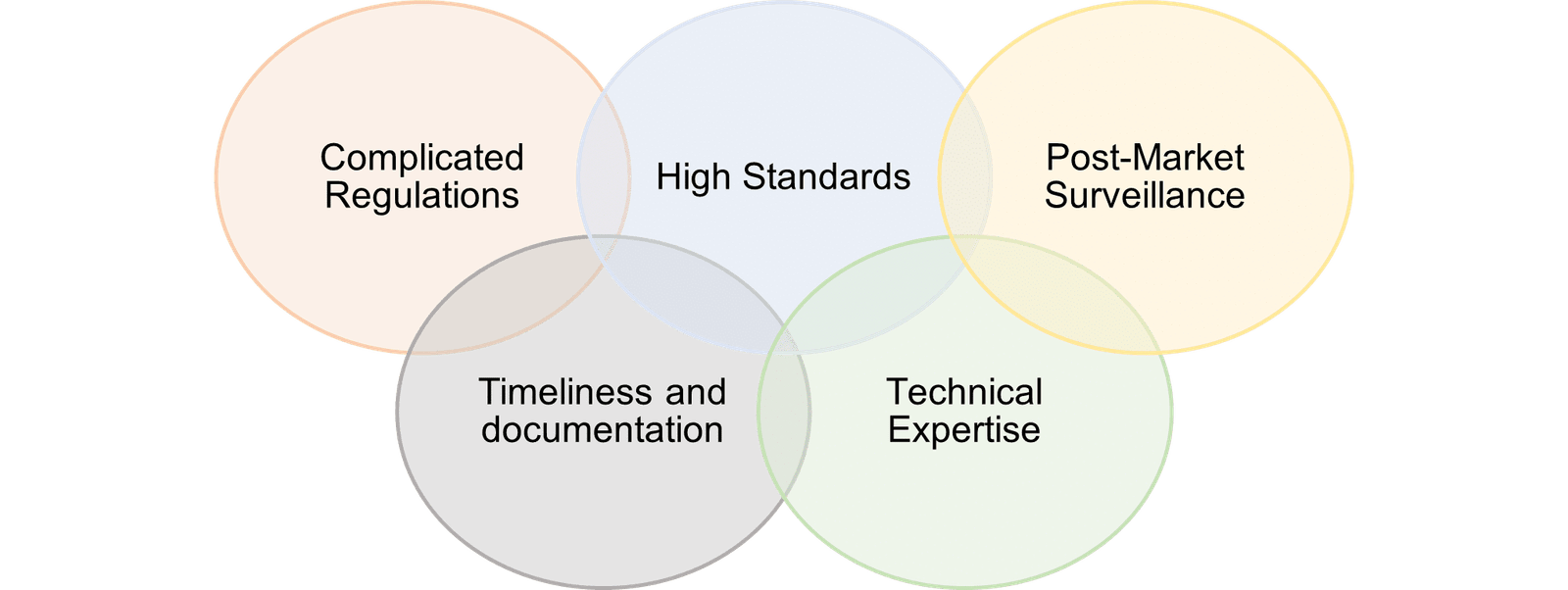
Challenges of making Product registration and PPB approved site in India
Conclusion
In summary, navigating the complex regulatory landscape is crucial for obtaining PPB Kenya accreditation and registering pharmaceutical products and manufacturing facilities. Despite challenges like lengthy approval processes and strict documentation, compliance ensures the safety, efficacy, and quality of healthcare products in Kenya. By actively engaging with regulatory authorities and investing in compliance, pharmaceutical businesses can overcome these obstacles and contribute to improved public health while accessing the growing Kenyan market.
This article was crafted under the expert guidance of Ms. Nidhi Patel, showcasing her invaluable contribution to its preparation and quality. – Team Conical Pharmaceuticals