EU GMP: What is it?
EU GMP is Good Manufacturing Practices, promulgated by the European Medicines Agency (EMA). EU GMP is the guidelines that pharma manufacturers must meet in their medicine manufacturing process.
What is EMA?
EMA is European Medicines Agency, established in 1995. The head office is in Amsterdam, the Netherlands.
EMA is an agency of the European Union (EU) whose main role is to evaluate, authorize and monitor medicines for human and veterinary, circulating in the European Union and the Economic area. Europe. Medicines evaluation, authorization, and monitoring help ensure the safety and health of people and animals in Europe.
What is EU GMP certificate?
GMP certificate is granted on the basis of GMP inspection results for production facilities operating in accordance with EU GMP standards.
The certificate is issued when all dossiers and documents about the inspection are submitted.
The EU GMP Certificate declares the manufacturer’s compliance with EU GMP standards. On the Certificate, reference to the date, month, and year of the most recent inspection, as well as the activities and legal basis are included.
Objective
The objective is to ensure product quality and potency, as well as patient safety, by minimizing risks of microbial, particulate and endotoxin/pyrogen contamination through manufacturing and environmental controls.
How to achieve EU GMP certificate For Plant Registration in India?
Before entering the EU market, companies from regions such as the CIS, Middle East, Asia, or Latin America must secure an EU GMP certificate for their manufacturing facility or contract manufacturer.
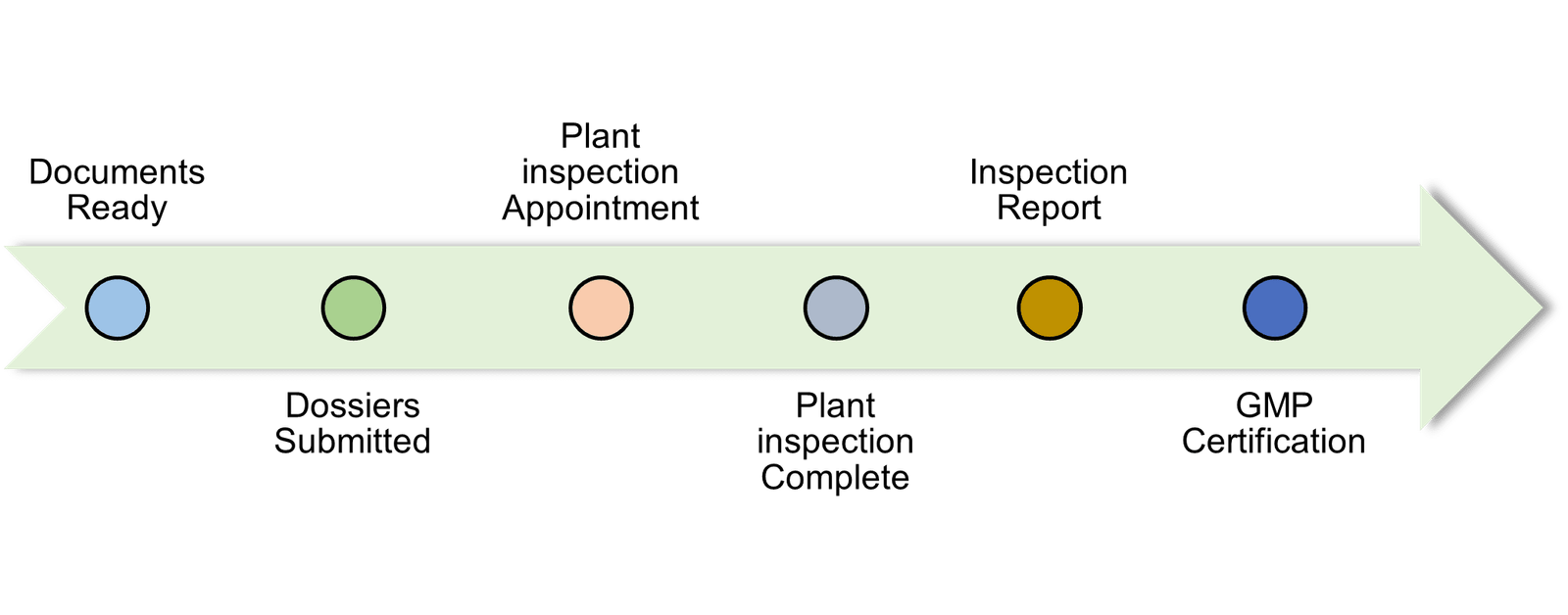
To apply for this certification, pharmaceutical companies need to follow these six essential steps:
- Prepare administrative documents and production locations
- Submit your application to the licensing authority in Europe
- Arrange factory inspection appointments
- Organize factory inspection by licensing agency
- Purchase inspection report and GMP certification
- Achieve EU GMP certificate
Validity of EU GMP Approved Manufacturer Certificate
A GMP certificate is valid for three years from the most recent date of inspection, but the period of validity can be shortened under special circumstances. Since a GMP certificate refers to a specific inspection, it cannot be renewed or reissued until a new inspection is conducted and completed with adequate follow-up.
Product registration process for EU GMP Approved manufacturer in India
-
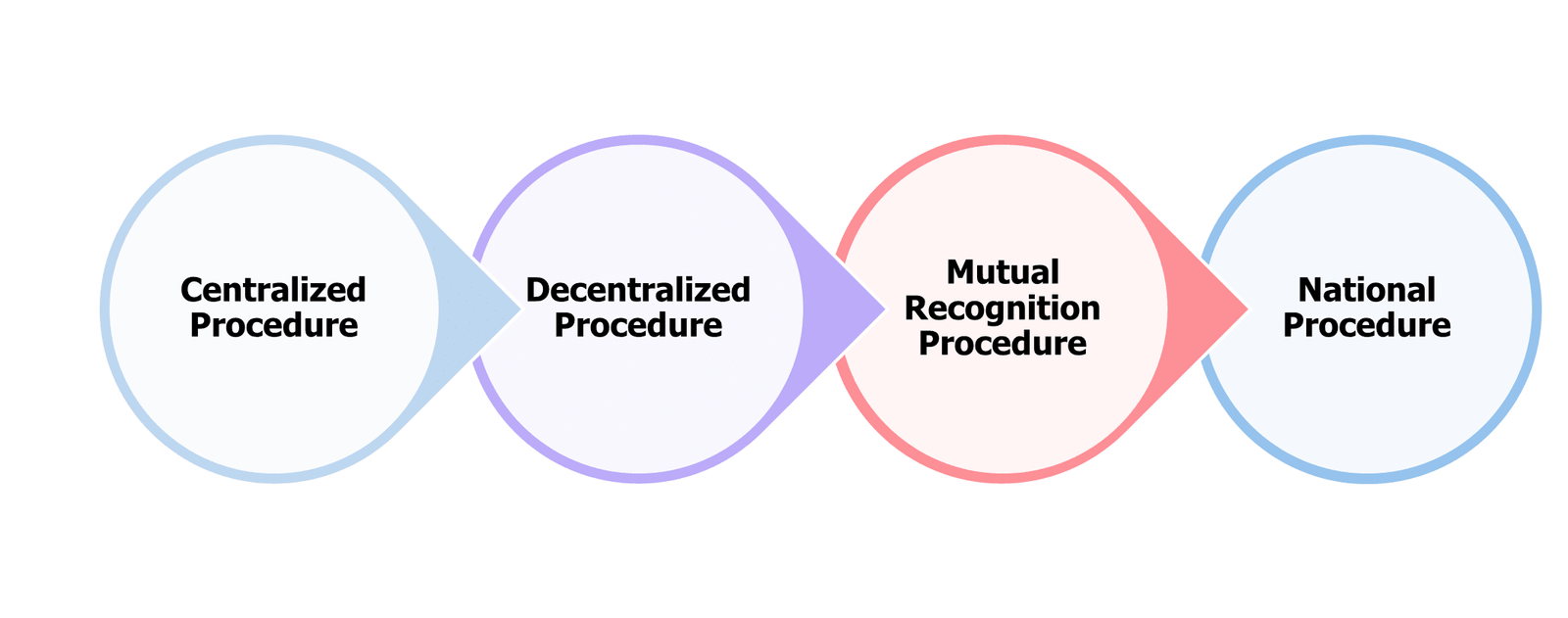
Centralized Procedure
The centralized procedure is used for the marketing authorization of medicinal products, requiring a single application, evaluation, and authorization to be valid across all EU member states. This process allows the product to be marketed throughout the entire EU market. Applications for this authorization are submitted directly to the European Medicines Agency (EMA).
-
Decentralized Procedure
While a pharmaceutical product should not be approved in any member state at the time of application, this technique is utilized to get marketing authorization in multiple member states.
-
Mutual Recognition Procedure
When a medicinal product is already permitted in any member state at the time of application, this approach is used to seek marketing authorization in several member states. An applicant may request a marketing permission from one or more member states following the community’s first approval of the medicinal product. It is simpler to obtain marketing permission for a pharmaceutical product in subsequent member states after obtaining the first one, as the product’s existence on the European market serves as evidence of its therapeutic efficacy.
-
National Procedure
Applications for marketing authorization should be submitted to the national competent authority of the specific member state where the applicant wishes to get authorization for only one of its member states. In this instance, a pharmaceutical product should not receive approval for national marketing in another member state under the same sponsor.
Product Registration fees for EU GMP Approved Manufacturer in India
The Agency charges fees for applications for marketing authorisation, and for variations and other changes to marketing authorisations, as well as annual fees for authorised medicines.
Examples of current basic fees
| Fee type | Human medicines |
| Marketing-authorisation application (single strength, one pharmaceutical form, one presentation) | From €345,800 |
| Extension of marketing authorisation (level I) | €103,800 |
| Type-II variation (major variation) | €103,800 |
| Annual fee (level I) | €123,900 |
Facing challenges of getting EU GMP Certificate and Product approval
Conclusion
Obtaining EU GMP certification and navigating the product registration process is essential for pharmaceutical companies looking to enter the European market. While the process can be complex and challenging, it ensures compliance with high manufacturing standards, improves product quality, and opens up opportunities for wider market access. Despite the associated costs, the benefits of EU GMP certification, such as enhanced competitiveness and credibility, make it a vital step for success in the global pharmaceutical industry.
References: EU GMP (European Union level) Certification & Approval Process
This article was crafted under the expert guidance of Ms. Nidhi Patel, showcasing her invaluable contribution to its preparation and quality. – Team Conical Pharmaceuticals