Understanding INVIMA’s Role
In the dynamic landscape of pharmaceuticals, ensuring the safety, efficacy, and quality of drugs is paramount. For pharmaceutical manufacturers and exporters, obtaining certification and approval from regulatory bodies like the National Institute of Food and Drug Surveillance (INVIMA) in Colombia is crucial. INVIMA Colombia, founded in 1994 serves as Colombia’s primary regulatory body for medicines, medical devices, dietary supplements, cosmetics, and food products. Its mandate encompasses a wide range of responsibilities, including product registration, quality control, and enforcement of regulatory standards.
INVIMA Certified Manufacturers and Suppliers
INVIMA requires pharmaceutical manufacturers and suppliers to undergo a rigorous certification process to ensure compliance with Good Manufacturing Practices (GMP). Being a certified manufacturer or supplier signifies adherence to international quality standards, guaranteeing the production of safe and effective pharmaceutical products. By obtaining certification from INVIMA, manufacturers and suppliers demonstrate their commitment to quality and consumer safety, paving the way for approved drug registration and exportation.
Manufacturing Plant Registration for INVIMA Certification
In addition to drug registration, pharmaceutical manufacturing plants must undergo registration with INVIMA. Plant registration involves a thorough assessment of manufacturing facilities, equipment, and processes to ensure compliance with GMP standards. By registering their plants with INVIMA, manufacturers demonstrate their commitment to quality assurance and regulatory compliance. Plant registration is a prerequisite for drug registration, as products can only be marketed if they are manufactured in registered facilities.
For INVIMA site registration under Good Manufacturing Practices (GMP), the following documents are typically required:
- Site Master File (SMF)
- Quality Manual
- Standard Operating Procedures (SOPs)
- Validation Master Plan (VMP)
- Validation Protocols and Reports
- Batch Records
- Equipment Qualification Documentation
- Cleaning Validation Documentation
- Change Control Procedures
- Training Records
- Quality Control Records
- Deviation Reports
These documents, along with any additional requirements specified by INVIMA, demonstrate the site’s compliance with GMP standards and regulatory requirements for pharmaceutical manufacturing.
INVIMA Site Certification for Manufacturing Plants
Site certification is another essential aspect of pharmaceutical manufacturing and distribution. Sites involved in drug storage, distribution, and packaging must comply with GMP standards and undergo certification by INVIMA. Certified sites ensure the integrity and quality of pharmaceutical products throughout the supply chain, from manufacturing to distribution. By certifying sites, INVIMA upholds safety standards and protects consumers from counterfeit or substandard drugs.
Exporter Compliance for INVIMA Regulations
For pharmaceutical exporters, compliance with INVIMA regulations is paramount to ensure market access and consumer trust. Exporters must ensure that their products are registered with INVIMA and sourced from certified manufacturers and suppliers. By adhering to regulatory requirements, exporters demonstrate their commitment to quality and compliance, enhancing their reputation in international markets.
Pharmaceutical Regulations and Registration Process for Getting INVIMA Approval
The following steps are involved in registering a pharmaceutical product with INVIMA in Colombia:
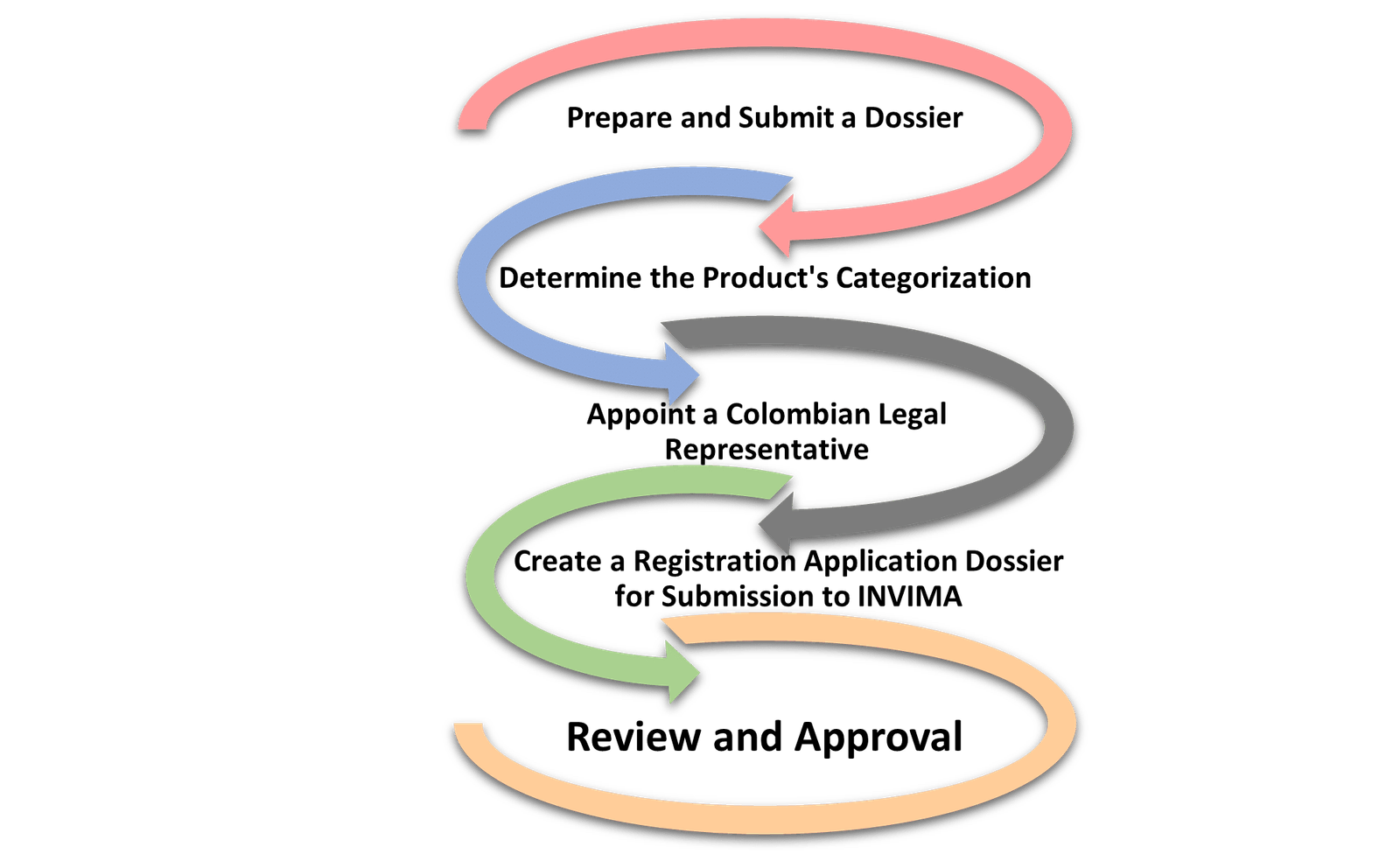
A. Health Authority
Colombia’s regulatory body for medical devices and pharmaceuticals registration and regulations is the National Food and Drug Surveillance Institute (INVIMA). INVIMA plays a vital role in safeguarding the safety, effectiveness, and quality of healthcare products. It takes charge of critical functions such as supervising clinical trials, managing registrations, and conducting post-market surveillance.
B. Legal Representation
In Colombia, to be INVIMA approved manufacturers and importers of medical devices and pharmaceuticals must appoint an authorised representative. This representative serves as a bridge between the companies and the regulatory authorities, ensuring compliance with regulations and facilitating communication.
C. Product Classification
In Colombia, medical devices and pharmaceuticals undergo classification based on the level of potential risks they pose. The country follows a four-class system for medical devices (Class I, Ila, Ilb, and III) and a similar classification for pharmaceuticals. This categorization helps to ensure appropriate regulatory oversight and safety measures.
- Class I Medical Device: Bandages, non-invasive thermometers.
- Class Ila Medical Device: Hearing aids, syringes.
- Class IIb Medical Device: Surgical lasers, infusion pumps.
- Class III Medical Device: Implantable pacemakers, heart valves.
- Pharmaceutical Class A: Over-the-counter painkillers.
- Pharmaceutical Class B: Prescription antibiotics.
- Pharmaceutical Class C: High-risk medications like immunosuppressants.
D. Labeling Requirements
When it comes to labelling, it is crucial for medical devices and pharmaceuticals in Colombia to include essential information. This includes the product name, details of the manufacturer, instructions for usage, important precautions, and regulatory information. It is mandatory for this information to be provided in both Spanish and the local language to ensure effective communication and understanding by users.
E. Clinical Trial Requirements
In Colombia, clinical trials undergo a rigorous approval process by INVIMA, the national regulatory authority for medicines and medical devices. These trials must adhere to ethical and scientific standards to ensure patient safety and reliable results. Detailed documentation, such as the study protocol and informed consent forms, is crucial for the successful initiation and execution of clinical trials in the country.
F. Testing Requirements
Medical devices and pharmaceuticals go through a thorough and rigorous testing process to ensure that they are safe and effective for use. These tests involve several important aspects, including biocompatibility, stability, performance, and toxicity studies.
G. Foreign Testing and Recognition
Colombia typically accepts testing data from foreign sources, but there might be additional requirements such as translation and validation. Notably, testing data from countries like the European Union (EU) and the United States (US) is often recognized in Colombia.
H. Pre-registration Requirements
In Colombia, to be INVIMA approved manufacturers and importers of medical devices or pharmaceuticals are required to obtain an establishment license from the Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) before they can register their products. This license is an important step in ensuring compliance with Good Manufacturing Practices (GMP). Obtaining this license demonstrates the manufacturer’s or importer’s commitment to maintaining high manufacturing standards and delivering safe and effective products to the Colombian market.
I. Registration Process to be INVIMA Approved Manufacturer
During the registration process in Colombia, manufacturers or importers are required to provide a comprehensive set of technical and regulatory documents for their products. These documents provide detailed information about the composition, manufacturing process, labelling, and clinical data of the product. The purpose of this requirement is to ensure that the product meets the necessary standards and regulations for safety and efficacy. By providing these documents, manufacturers and importers enable the regulatory authorities to thoroughly evaluate the product before granting approval for it to enter the market. This process helps to safeguard public health and ensures that only compliant and well-documented products are available to consumers in Colombia.
J. Expedited Approval based on EU/US Approval
Obtaining approval for a product in the European Union (EU) or the United States (US) can greatly facilitate the registration process in Colombia. This is because these jurisdictions are recognized for their stringent regulatory requirements and high standards. When a product is already approved in the EU or US, it indicates that it has undergone a thorough evaluation and meets rigorous criteria for safety and efficacy.
K. Import and Distribution Requirements
In Colombia, it is mandatory for importers to obtain an import license from the National Institute for Food and Drug Surveillance (INVIMA). This license enables importers to legally bring their products into the country. Additionally, distribution centres in Colombia must adhere to Good Storage Practices (GSP). These practices are designed to maintain the quality and integrity of products during their storage and transportation.
L. List of Documents Required
The specific documents required include:
- Product technical dossier
- Manufacturing process details
- Clinical trial data
- Proof of GMP compliance
- Labelling and packaging information
- GMP Inspection Requirements:
Manufacturers and facilities are subject to GMP inspections by INVIMA to ensure compliance with manufacturing standards.
M. Timeline and Associated Fees for INVIMA Approval
The timeline for approval can vary based on factors such as the product’s classification and complexity.
| Registration | Official timeline for approval | Validity of marketing authorization |
| New drug products, included in Colombian pharmacological norms, foreign manufacturing | 80 Days | 5 years |
| New drug products, included in Colombian pharmacological norms, national manufacturing | 200 Days | 5 years |
Associated fees also differ according to the type of product and its classification. Government fees for marketing authorization in USD for various types of products are as follows:
– Drug Product in the Pharmacological Code: $3,200
– New drug: $7,000
– Biological Products: $7,238
– Good Manufacturing Practice Certificate: $12,035 USD.
N. Post-Marketing Activities
- Pharmacovigilance involves the continuous monitoring and reporting of any adverse events that may be associated with pharmaceutical products.
- Variations refer to the process of submitting changes to already approved products, such as adjustments to their manufacturing processes.
- Renewal refers to the periodic renewal of product registrations to ensure their continued compliance with regulatory standards.
- Manufacturers and importers may also be subject to audits as a means of ensuring ongoing compliance with regulations.
Challenges for drug and site registration with INVIMA include:
- Meeting stringent regulatory compliance
- Providing comprehensive documentation
- Ensuring adherence to quality standards
- Submitting clinical trial data
- Managing timelines and potential delays
- Addressing language and cultural differences
- Establishing local representation
- Managing costs associated with registration
- Implementing post-market surveillance
- Keeping up with policy changes and updates
Conclusion
In the realm of pharmaceuticals, certification and approval from regulatory authorities like INVIMA play a pivotal role in ensuring product quality, safety, and efficacy. Manufacturers, plants, sites, suppliers, and exporters must undergo rigorous certification processes to comply with GMP standards and regulatory requirements. By obtaining certification and approval from INVIMA, stakeholders demonstrate their dedication to upholding the highest standards of pharmaceutical manufacturing and distribution, ultimately benefiting consumers and public health.



