What is FDA Philippines?
The Food and Drug Administration (FDA) of the Philippines, established under Republic Act No. 3720 and later amended by Republic Act No. 9711, is responsible for ensuring the safety, efficacy, and quality of health products. This includes regulating food, drugs, cosmetics, medical devices, vaccines, and other health-related products such as pesticides and toys, as mandated by the Department of Health.
Mission
To guarantee the access of the general public to safe, quality, pure and efficacious health products through sound and innovative regulations.
Vision
A nation with well-informed consumers with access to regulated health products
An efficient regulatory agency providing modernized solutions in ensuring access to quality health products
Goals
To protect and promote the right to health of the Filipino people by ensuring the safety, efficacy, quality, and purity of foods, drugs, devices, and cosmetics, and
To establish and maintain an effective health products regulatory system responsive to the country’s health needs and problems.
Who Needs to Register with FDA Philippines?
In the Philippines, companies and entrepreneurs involved in importing, exporting, wholesale trading, distribution, or manufacturing FDA-regulated products must register with the FDA. They need to secure a License to Operate (LTO) and obtain a Certificate of Product Registration (CPR) for each product to legally conduct these activities.
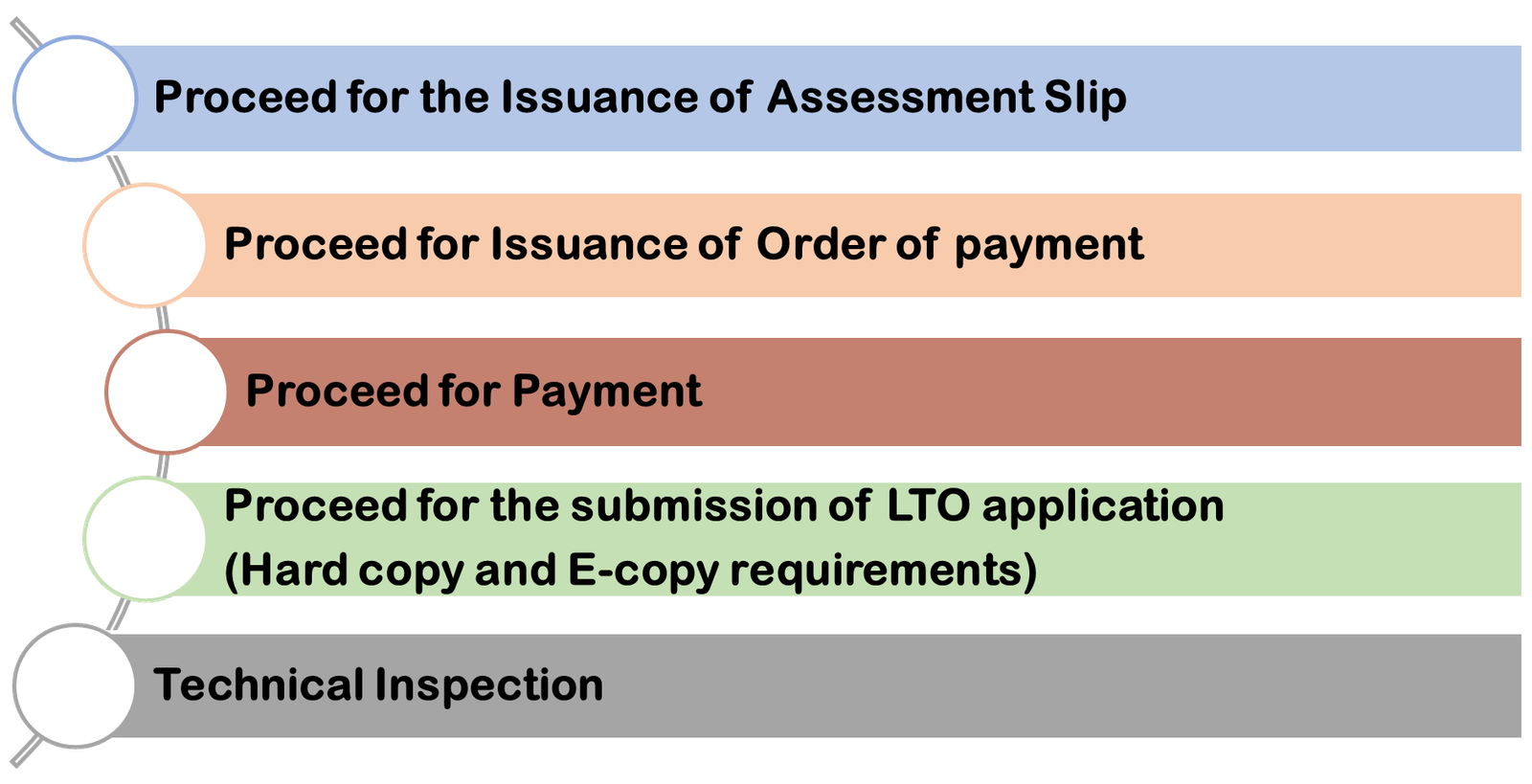
How To Obtain Philippines FDA Approved Site Registration Certificate in India
Requirements for LTO Application
- Petition document:
The petition form needs to be notarized and submitted by the owner, incorporator, or an authorized representative.
- Joint Affidavit of Undertaking:
An affidavit has to be signed by the company’s owner and a designated signatory. They are going to clarify what they know about the FDA’s LTO requirements. A professional in allied health sciences or pharmacy is an approved person. It is also required that they show their board certificate or professional license.
- Certificate of Attendance to an FDA-sponsored seminar on licensing of establishments:
The business owner or relevant authorized person must attend the seminar. It is also possible to submit a promissory letter to compensate the attendance.
- Proof of business registration:
It is, for example, your registration with the Securities and Exchange Commission. It is possible to submit your business permit from your local government unit.
- Proof of occupancy or business address:
Applicants must submit documents related to the business location. For example, the contract of lease for a rented space.
- Floor plan and vicinity map:
Documents pertaining to the actual business location are required of applicants. A floor layout with dimensions is included in the documentation.
Validity Of Certificate for Philippines FDA Approved Manufacturer in India
A business can submit an application for an FDA certificate at the local FDA regional field office or central office. The initial license is good for two years. The validity of renewed LTOs is five years.
Being A Philippines FDA Approved Site Registration and Licensing Requirements
| Regulatory Authority | Food and Drug Administration (FDA), Philippines |
| Website of regulatory Authority | https://www.fda.gov.ph/ |
| Fees for drug Registration | PhP 20,000.00 |
| Normal time taken for registration | 18 Months |
| Registration Requirement [Dossier Format] | ACTD |
| Whether plant inspection is mandatory | Yes |
| Validity of Registration | 05 Years |
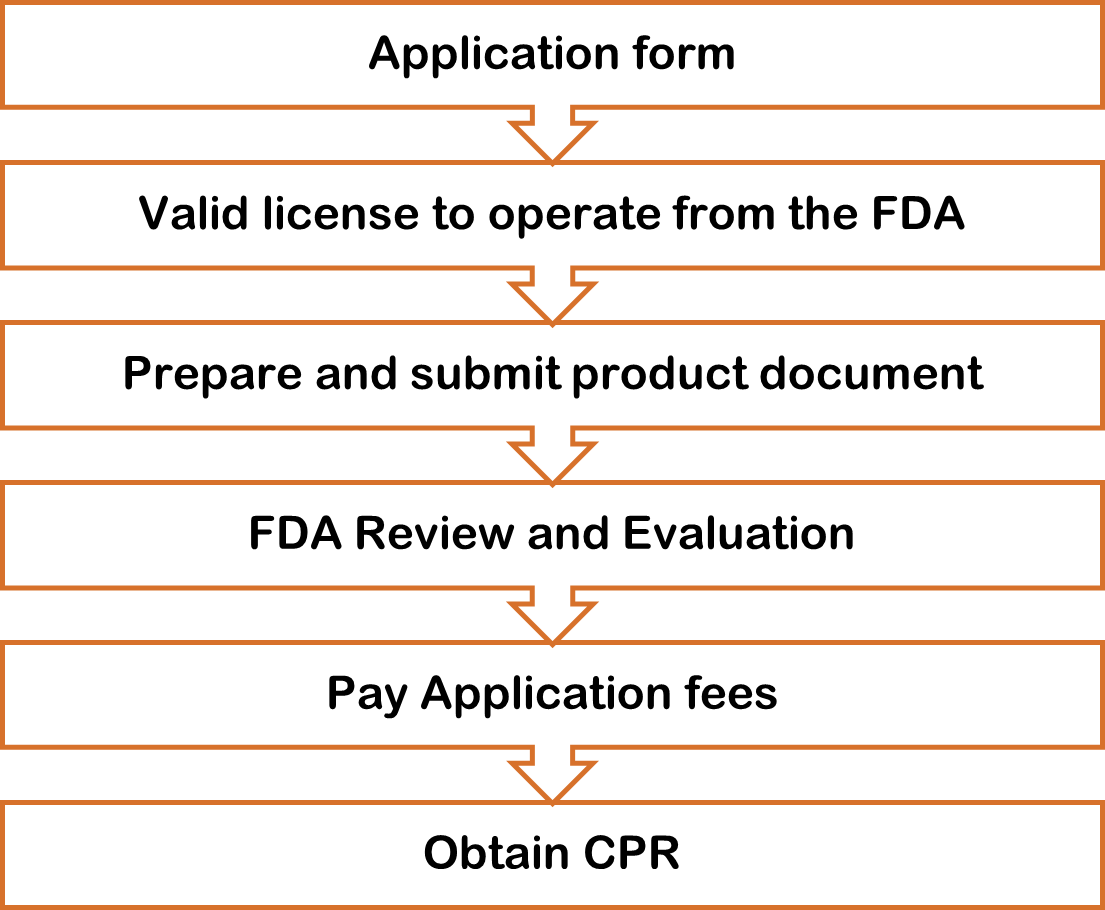
How To Apply Philippine FDA Approved Plant for Product Registration?
After getting your license to operate, you can apply for an FDA certificate of product registration. The FDA certificate is valid for 1-2 years and is subject to renewal upon expiration.
General Requirements for Dossier Preparation
- Part I: Table of Content, Administrative Information and Prescribing Information
- Part II: Quality Document
- Part III: Nonclinical Document
- Part IV: Clinical Document
| Parts | Requirements |
| Part I:
Table of Content, Administrative Information and Prescribing Information |
Certificate of Pharmaceutical Product
Free Sale Certificate Letter of Authorization Company certifications A general introduction of the pharmaceutical product, including its pharmacologic class and mode of action shall be included. |
| PART II:
Drug Substance (S) |
General Information
– Nomenclature – Structural Details – General Properties Manufacture – Manufacturer(s) – Description of Manufacturing Process and Process Controls – Control of Materials – Controls of Critical Steps and Intermediates – Process Validation and/or Evaluation – Manufacturing Process Development Characterizations – Elucidation of Structure and Characteristic – Impurities Control of Drug Substance – Specification – Analytical Procedures – Validation of Analytical Procedures – Batch Analyses Justification of Specification Reference Standards or Materials Container Closure System Stability Stability Summary and Conclusion |
| Drug Product (P) | Description and Composition
– Description of the dosage form Pharmaceutical Development – Information on Development Studies – Component of Drug Product – Active Ingredients – Excipients – Finished Product – Formulation Development – Overages – Physicochemical and biological Properties – Manufacturing Process Development – Container Closure System – Microbiological Attributes – Compatibility Manufacture – Batch formula – Manufacturing Process and Process Control – Controls of Critical Steps and Intermediates – Process Validation and/or Evaluation Control of Excipients – Specification – Analytical Procedures – Excipients of Human and Animal Origin – Novel Excipients Control of finished Product – Specification – Analytical Procedures – Validation of Analytical Procedures – Batch analyses – Characterization of Impurities Justification of Specification Reference Standards or Materials Container Closure System Stability – Stability Summary and Conclusion – Post-approval stability protocol and stability commitment – Stability Data Product Interchangeability |
| Part III: | It contains the Non-Clinical Documents having four sections namely:
Section A: contains the Table of content for the entire Part III, Section B: contains the Non clinical Overview means the summary of the Non clinical studies Section C: contains the written and Tabulated summaries of the Pharmacological, Pharmacokinetics and Toxicological data, Section D: contains the Nonclinical Study Report of Pharmacology, Pharmacokinetics and Toxicology data |
| Part IV: | It contains the Clinical Studies having Six Sections namely:
Section A: Table of Content for the entire Part IV Section B: comprises of Clinical Overview of the complete Clinical Studies documented in Part IV Section C: Summary of Biopharmaceutics and Associated Analytical Methods, Clinical Pharmacology Studies, Clinical Efficacy, Clinical Safety and Synopses of Individual Studies Section D: contains tabular listing of all clinical studies Section E: contains the Clinical Study Reports Section F: contains the list of Key References from where all the clinical Studies are Published and Documented. |
Costs Associated with Philippine FDA Approved Manufacturer for Product Registration
This schedule of fees is based on FDA Administrative Order
Product classification = PhP 500.00
New Drug Product = PhP 33,333.33
Amendment of CPR = PhP 200.00
Re-application Fees = PhP 200.00
Major variation = PhP 18,000.00
Minor variation = PhP 7,000.00
Conclusion
In conclusion, to effectively navigate the latest drug product registration protocols of the FDA in the Philippines, companies must prioritize staying informed, meticulously plan their strategies, and engage with regulatory experts to ensure accurate documentation and compliance.
This article was crafted under the expert guidance of Ms. Nidhi Patel, showcasing her invaluable contribution to its preparation and quality. – Team Conical Pharmaceuticals