What is the COFEPRIS?
COFEPRIS, the Federal Commission for the Protection against Sanitary Risks, is a pivotal regulatory body in Mexico, ensures the safety and quality of health products. Established in 2001 as mandated by Article 17 Bis of the General Law of Health and Article 4 of the Constitution, COFEPRIS is responsible for certifying and approving manufacturers, plants, and exporters involved in the production, importation, distribution, and marketing of pharmaceuticals, medical devices, food, cosmetics, and other health-related products.
Mission:
Protect the population against sanitary risks.
Vision:
Attain a healthy population properly protected against sanitary risks.
Organizational Structure
The Federal Commission is made up of eight administrative units and four government organs.
Administrative Units
- Evidence and Risk Management Commission
- Sanitary Promotion Commission
- Sanitary Authorization Commission
- Sanitary Operation Commission
- Analytic Control and Expansion of Coverage Commission
- General Coordination of the Federal Sanitary
- Legal and Consultative Coordination Center System
- General Secretariat
Government Organs
- Internal Council
- Scientific Council
- Mixed Consultative Council
- Consultative Publicity Council
Objectives
- Safeguarding Public Health
- Regulatory Compliance
- Risk Management
- Promoting Innovation
- Market Access
- Consumer Education
- International Collaboration
The Mandate of COFEPRIS
COFEPRIS is tasked with protecting the Mexican population from health risks associated with the consumption or use of health products. Its regulatory framework encompasses a wide range of responsibilities, including:
- Registration and Authorization
- Inspection and Surveillance
- Pharmacovigilance
- Market Surveillance
- Public Health Promotion
Navigating COFEPRIS: Certified Approval Process for Manufacturers
In the world of pharmaceuticals, getting your products approved by regulatory authorities like COFEPRIS is crucial. COFEPRIS certification is a rigorous process that evaluates manufacturing plants to ensure compliance with regulatory standards. As a manufacturer, gaining COFEPRIS approval signifies that your facility meets the stringent requirements set forth by the regulatory body.
A. Key Steps in the Certification Process for Manufacturing Plant

B. Benefits of COFEPRIS Certification
- Market Access: COFEPRIS certification allows manufacturers to legally sell their products in Mexico’s pharmaceutical market, enhancing market access and opportunities for growth.
- Quality Assurance: Certification demonstrates a commitment to quality and compliance, instilling confidence in consumers, healthcare professionals, and regulatory authorities.
- Global Recognition: COFEPRIS approval enhances a manufacturer’s reputation globally, facilitating export opportunities and partnerships with international suppliers.
Pharmaceutical Registration Process to be COFEPRIS Approved Manufacturer
The key steps in the medicinal product registration process in Mexico are highlighted below:

The process of registration with COFEPRIS starts with
Step 1: Consultation Meeting with COFEPRIS New Molecules Committee
A consultation meeting is mandatory before submitting the registration dossier to the New Molecule Committee (NMC) at COFEPRIS
- A meeting request must be submitted to NMC with the associated documentation.
- NMC reviews application and notifies applicant of meeting request decision within 60 days.
- NMC will notify the applicant of the scheduled meeting day 20 business days in advance.
- Applicant must Confirm scheduled meeting date with the NMC
- A presentation must be sent to NMC 5 days prior to the confirmed meeting date
- NMC will issue a *Technical Opinion 20 business days after the meeting.
- The applicant can submit the Technical Opinion and the complete Technical dossier to COFEPRIS
*A good technical opinion must be obtained in order to continue to Step 7 – COFEPRIS submission. If a technical opinion obtained advises the applicant for further revision the applicant must update their application and begin the application process to the NMC all over again.
Document Considerations:
- Supporting documents must be submitted in a USB drive.
- Documents must be Translated into Spanish.
- The applicant must identify the applicable evaluation route for the product registration.
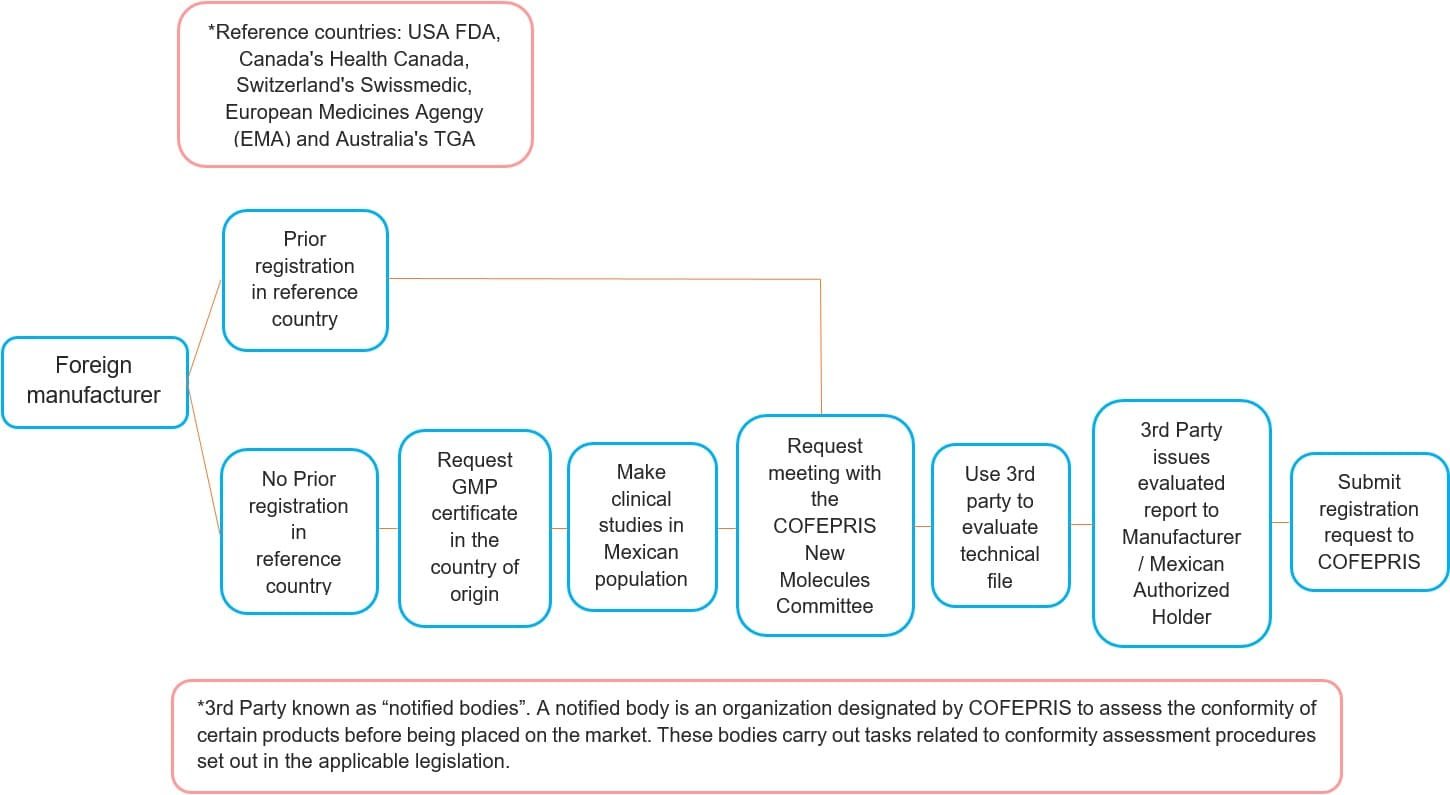
Step 2: Request GMP inspection from COFEPRIS
- Mexico has its own Good Manufacturing Practices requirements.
- Manufacturer must request COFEPRIS for GMP inspection before submitting the registration dossier.
- Manufacturer with GMP certificate from reference countries are exempt from COFEPRIS GMP inspection.
- GMP certification must be obtained for all applicable manufacturing sites. (drug substance and finished products).
Content of Application for GMP Inspection
- Name and general information of the requesting establishment
- Name of the drug or medicine for which you are requesting verification of GMP
- Name and full address of the establishment(s) involved in each stage of manufacturing
- Description of the process that is carried out in each of the establishments involved
- The manufacturing process for which verification of GMP is requested
- A list and description of products that are made.
- Name of the legal representative, health officer or person designated by the establishment to attend the diligence
- Organization charts (general, of the production and quality departments, indicating the reporting lines)
- Plans of the establishment and production areas
- Block diagram of the manufacturing process
- General summary of the quality system including validation and qualification
- Information from the last two annual review reports, specifically indicating: manufactured lots, rejected lots (indicating reasons), released lots that were subject to investigation, conclusion and actions carried out, number of reprocessed batches, complaints, returns and withdrawal of products from the market, as well as conclusions of the report.
Step 3: Prepare and Submit registration dossier to third party or directly to COFEPRIS for review and approval
Content of Registration Dossier
| Module No. | Content |
| Module I. Legal administrative information |
|
| Module II. Quality information |
API
Finished product
|
| Module III. Preclinical studies |
Preclinical studies
|
| Module IV. Clinical studies |
Clinical studies
|
Registration Process for Foreign Manufacturers
Required Registration Fees & Timelines for COFEPRIS Certified Manufacturer
| Classification |
Time of response (Natural Days) |
Fees (Mexican Pesos) |
| Generic | 180 Days | $82,011.99 |
| New molecule | 180 days | $146,641.88 |
|
Additional Fees |
Time of response (Business Days) |
Fees (Mexican Pesos) |
| GMP Inspection | Timelines Vary | $96,666.39 |
| Request meeting with the COFEPRIS New Molecules Committee | 60 days | NA |
| Receive New molecule Committee conclusions after meeting | 20-40 Days | NA |
*Fees for Notified Bodies Vary
Challenges of navigating the medicinal product Regulatory landscape in Mexico
- Complex and evolving regulations
- Limited guidance documents
- Long approval timelines
Conclusion
COFEPRIS, the certified regulatory authority in Mexico, plays a pivotal role in safeguarding public health by overseeing compliance with approved standards. As the primary regulator for health products, COFEPRIS ensures that manufacturers, plants, and sites adhere to stringent guidelines. By monitoring suppliers and exporters, COFEPRIS maintains the integrity of the supply chain, guaranteeing access to safe and effective health products for all Mexicans.
References: COFEPRIS – Mexico Ministry of Health
This article was crafted under the expert guidance of Ms. Ritika Patel, showcasing her invaluable contribution to its preparation and quality. – Team Conical Pharmaceuticals